Abstract
Venous thromboembolism is a well-known complication of sickle cell disease1-3 and is associated with significant morbidity and mortality, particularly in the case of pulmonary embolisms. On the other hand, concerns have been raised regarding the overuse of imaging when evaluating for PE.4,5 Multiple algorithms have been developed, including the Geneva score,6 Wells' criteria,7 and Pulmonary Embolism Rule-Out Criteria (PERC),8 to better determine the pre-test probability of pulmonary embolism and help curtail the utilization of imaging. Patients with sickle cell disease, particularly when in vaso-occlusive crisis, may have a presentation that confounds the data incorporated into the algorithms, such as abnormal vital signs or d-dimer measurements, making the evaluation challenging. Furthermore, acute chest syndrome (ACS) is another notable acute clinical manifestation of sickle cell disease and can have symptoms and objective measures that are similar to PE with PE itself being a potential trigger for ACS. There is limited data on the extent to which SCD patients undergo scans to evaluate for PE and on the yield of these scans. While complications of SCD including ACS have been found to be more common in patients with hemoglobin SS than SC or sickle beta thalassemia,9 venous thromboembolism has been found to be more prevalent in SC and sickle beta thalassemia.2
The sickle cell center at Grady Memorial Hospital, Atlanta, GA currently has more than 1,100 adult patients enrolled, accounting for the largest adult sickle cell population in North America. Through a retrospective chart review of these patients, we determined the prevalence of use of imaging to evaluate for pulmonary embolism and the yield from these radiographic studies, comparing this data across the hemoglobin genotypes. We also assessed for how often these these patients were ultimately diagnosed with ACS instead of or in addition to PE.
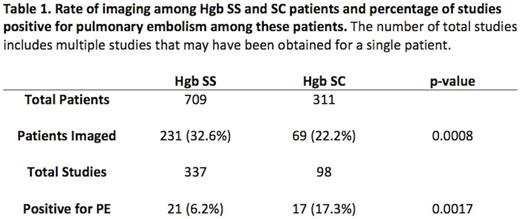
In total, the charts of 1,143 patients were reviewed. 1,127 of these patients had clearly documented hemoglobin (Hgb) phenotypes; the 16 charts without known Hgb phenotype were excluded from further analysis. Of the 1,127 patients, 709 patients were Hgb SS, 311 SC, 87 sickle beta plus thalassemia (Sb+), 18 sickle beta zero thalassemia (Sb°), 1 SD, and 1 O-Arab. 231 (32.6%) of the Hgb SS patients had imaging while 69 (22.2%) of the SC patients (Table 1), 20 (23.0%) of the Sb+ patients, and 3 (16.7%) of the Sb° patients had imaging. SS patients had 21 (6.2%) studies positive for PE while SC patients had 17 (17.3%) (Table 1), Sb+ patients had 4 (13.3%), and Sb° patients had 2 (9.1%) such studies. Of the PEs diagnosed via CT scans, 13 out of the 17 positive studies for SS patients involved segmental vessels, which was the case with 11 out of 15 for SC patients. When imaged, either inpatient or outpatient, 81 (24.0%) of the cases involving SS patients resulted in a diagnosis of acute chest syndrome; this occurred in 15 (15.3%) of SC patient cases, 4 (13.3%) of Sb+ cases, and 1 (4.5%) of Sb° cases.
In this study, we demonstrate that a significantly higher proportion of the Hgb SS patients underwent radiographic studies to evaluate for a pulmonary embolism compared to their SC counterparts despite having a significantly lower yield on these studies. However, the SS patients were more likely to be diagnosed with ACS, suggesting that this diagnosis may need to be more seriously considered before PE in this cohort. This data also suggests that though literature may have previously suggested that thromboembolic disease is more prevalent in SC than SS patients, there may be less awareness of such data among providers, who may be assuming that all sickle cell complications are most common in SS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal